43 fda approved health claims on food labels
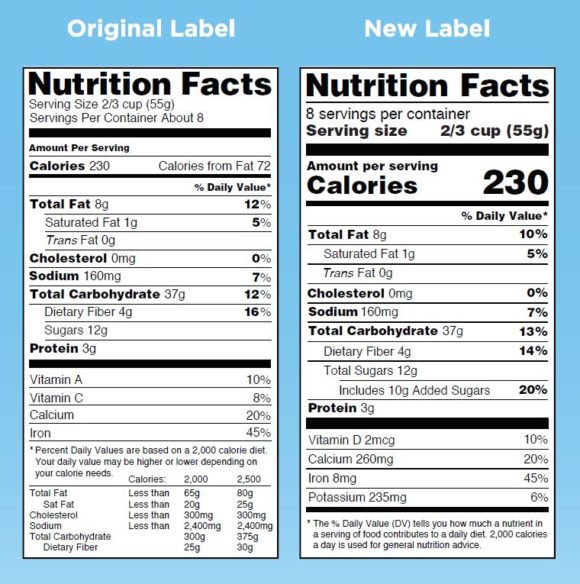
How to Read Food Labels: Understanding Claims & Components ... Reading a Food Label. The FDA has proposed some changes to the current nutrition facts label. We see both the old and the proposed new label here. ... For a health claim to be approved, it must be ... EOF
Authorized Health Claims That Meet the Significant ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...

Fda approved health claims on food labels
2.6: Understanding Food Labels - Medicine LibreTexts Food companies are allowed to make certain claims related to health and disease on food labels. A health claim is a statement that links a particular food with a reduced risk of developing disease. As such, health claims such as "reduces heart disease," must be evaluated by the FDA before it may appear on packaging. › news-events › public-health-focusFDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20, extralabel use of an approved human drug in a food-producing animal is not permitted if an animal drug approved for use in food-producing animals can be used in ... CFR - Code of Federal Regulations Title 21 - Food and Drug ... (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
Fda approved health claims on food labels. › consumers › consumer-updates5 Things to Know About Triclosan | FDA Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332) Reading Food Labels - What You Need to Know Health Claims: Food labels may have a message that tells how a food or part of a food affects a disease or a health condition. The United States Food and Drug Administration (FDA) has approved certain health claims that can be made on foods. It is important to read food labels very carefully. Some foods may list health claims that have not been ... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Understanding the USDA Organic Label | USDA Amidst nutrition facts, ingredient lists, and dietary claims on food packages, "organic" might appear as one more piece of information to decipher when shopping for products. Understanding what the organic label means can help shoppers make informed purchasing choices. Organic is a labeling term found on products that have been produced using cultural, biological, and mechanical practices ...
All of the following are FDA approved health claims ... Weegy: Diets that contain plentiful sources of potassium may reduce the risk of high blood pressure and stroke.User: What type of claim must be supported by scientifically valid evidence for it to be approved for use on a food label ] Weegy: Health claim must be supported by scientifically valid evidence for it to be approved for use on a food label. 13 Misleading Food Label Claims and How Not to Be Tricked 1. Label Says "Sugar-Free". The Food and Drug Administration (FDA) provides guidelines for a variety of common food labels, including sugar-free. While the term suggests that products labeled this way would be completely free of sugar, they can actually contain up to 0.5 grams of sugar in a single serving size. CFR - Code of Federal Regulations Title 21 - Food and Drug ... One or more model health claims that represent label statements that may be used on a food label or in labeling for a food to characterize the relationship between the substance in a food to a... CFR - Code of Federal Regulations Title 21 - Food and Drug ... (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
› news-events › public-health-focusFDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20, extralabel use of an approved human drug in a food-producing animal is not permitted if an animal drug approved for use in food-producing animals can be used in ... 2.6: Understanding Food Labels - Medicine LibreTexts Food companies are allowed to make certain claims related to health and disease on food labels. A health claim is a statement that links a particular food with a reduced risk of developing disease. As such, health claims such as "reduces heart disease," must be evaluated by the FDA before it may appear on packaging.

Ishigaki Glutathione Now FDA Approved | Dear Kitty Kittie Kath- Top Lifestyle, Beauty, Mommy ...










Post a Comment for "43 fda approved health claims on food labels"